Welcome to states of matter world where the ordinary becomes extraordinary, where the invisible becomes visible, and where the secrets of matter are unlocked.
Have you ever wondered how every object we use or encounter in our daily lives is made up?
Every object is made up of some types of material and on the other hand material is made of matter.
We can say, matter constitutes the building blocks of various materials, and these materials make up different objects.
But how can we make this complex topic come alive in the classroom?
Look no further! Our collection of engaging worksheets is here to shake up your science class and transform learning into an exhilarating adventure.
Our worksheets will help children to learn about matter and its states, difference between states, and change from one state to another.
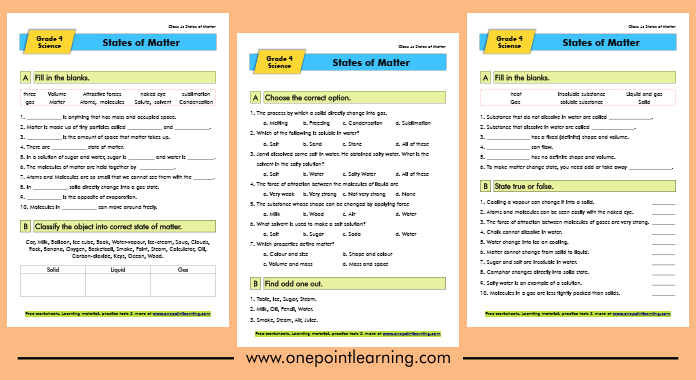
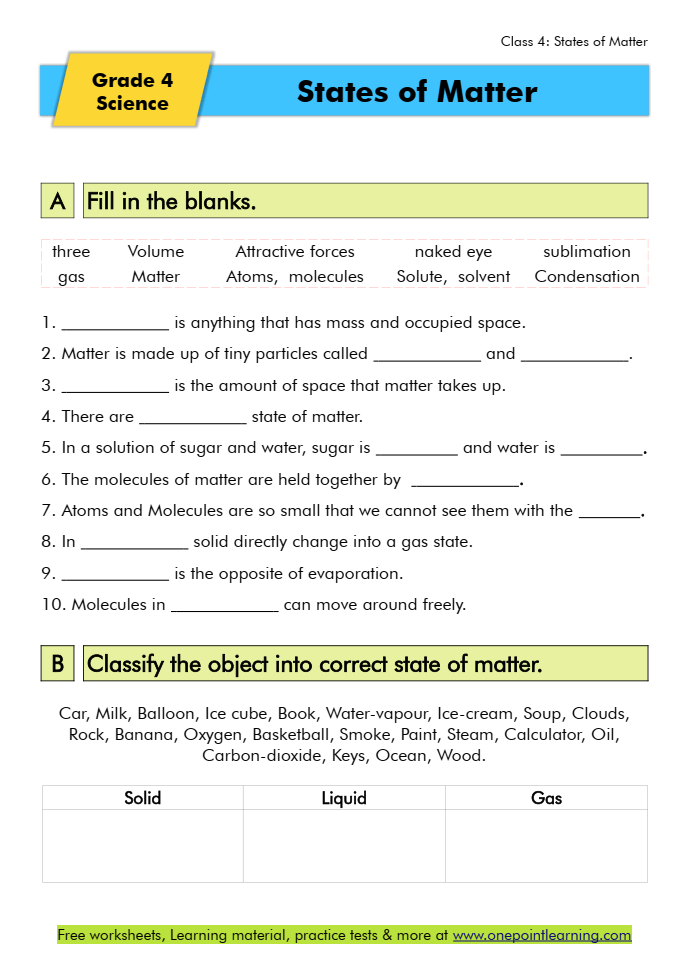
Download our specially designed States of Matter Worksheets for class 4 to boost your child’s confidence.
States of Matter Worksheets PDF
Also Check:
Science Worksheets for Class 4
Maths Worksheets for Class 4
Importance of Understanding States of Matter
Understanding states of matter is an essential building block in any science curriculum. It lays the foundation for comprehending the physical properties and behaviour of materials.
By grasping the concepts of solids, liquids, and gases, students gain a deeper understanding of how matter behaves and interacts with its surroundings.
Moreover, studying states of matter helps students develop critical thinking skills. It encourages them to observe, analyse, and draw conclusions based on their observations. This ability to think critically is indispensable in scientific inquiry and problem-solving.
Understanding Matter?
Matter is everything around us that takes up space and has mass. It includes everything we can see and touch, like people, animals, plants, rocks, water, air, and even the smallest particles that make up everything else.
Matter is made up of tiny particles called atoms and molecules that are too small to see with the naked eye.
Overview of the Three States of Matter – Solid, Liquid, Gas
To understand states of matter, we must first explore the three primary states: solid, liquid, and gas. Each state exhibits distinct characteristics and properties that set them apart from one another.
A solid is a state of matter characterized by a fixed shape and volume. The particles in a solid are densely packed and vibrate in place. They have a strong intermolecular force, which gives solids their rigidity and resistance to deformation. Examples of solids include rocks, metals, and ice.
On the other hand, a liquid is a state of matter that flows and takes the shape of its container. Liquids have a definite volume but no fixed shape. The particles in a liquid are close together but can move past each other, allowing the liquid to flow. Common examples of liquids are water, oil, and milk.
Lastly, a gas is a state of matter that has neither a fixed shape nor volume. Gaseous particles are far apart and move rapidly in all directions. They have weak intermolecular forces, which allows gases to expand and fill any container they are placed in. Air, oxygen, and carbon dioxide are examples of gases.
Exploring Changes in States of Matter – Melting, Freezing, Evaporation, Condensation

Changes in states of matter occur when energy is added or removed from a substance. These changes are known as phase transitions and involve the conversion between solid, liquid, and gas states.
Melting is the process of changing a solid into a liquid by adding heat energy. The solid particles absorb energy, increasing their kinetic energy and breaking the intermolecular forces holding them together. This results in the solid melting into a liquid.
Freezing, on the other hand, is the opposite of melting. Freezing is the process of changing a liquid into a solid by removing heat energy. The liquid particles lose energy, decreasing their kinetic energy and forming intermolecular forces, causing the liquid to solidify.
Evaporation occurs when a liquid changes into a gas. It happens when the particles in the liquid gain enough energy to overcome the intermolecular forces and escape into the air. Evaporation is influenced by factors such as temperature and surface area.
Condensation is the reverse of evaporation. It is the process of a gas changing into a liquid. When a gas loses energy, its particles slow down and come closer together, forming intermolecular forces and turning into a liquid.
Conclusion and Final Thoughts
States of matter are the building blocks of our physical world. By understanding the properties and behaviour of solids, liquids, and gases, we gain insights into the fundamental nature of matter.
Learning states of matter in an engaging and interactive way not only fosters a deeper understanding, but also ignites a passion for scientific exploration.
What do you get in States of matter Worksheet
- Printable science Worksheet in A4 PDF format.
- Free to download and easy to print at home.
- Answer key available at the last page.
- Wholly based on latest syllabus and pattern.
- Progressive approach with increasing difficulty level.

